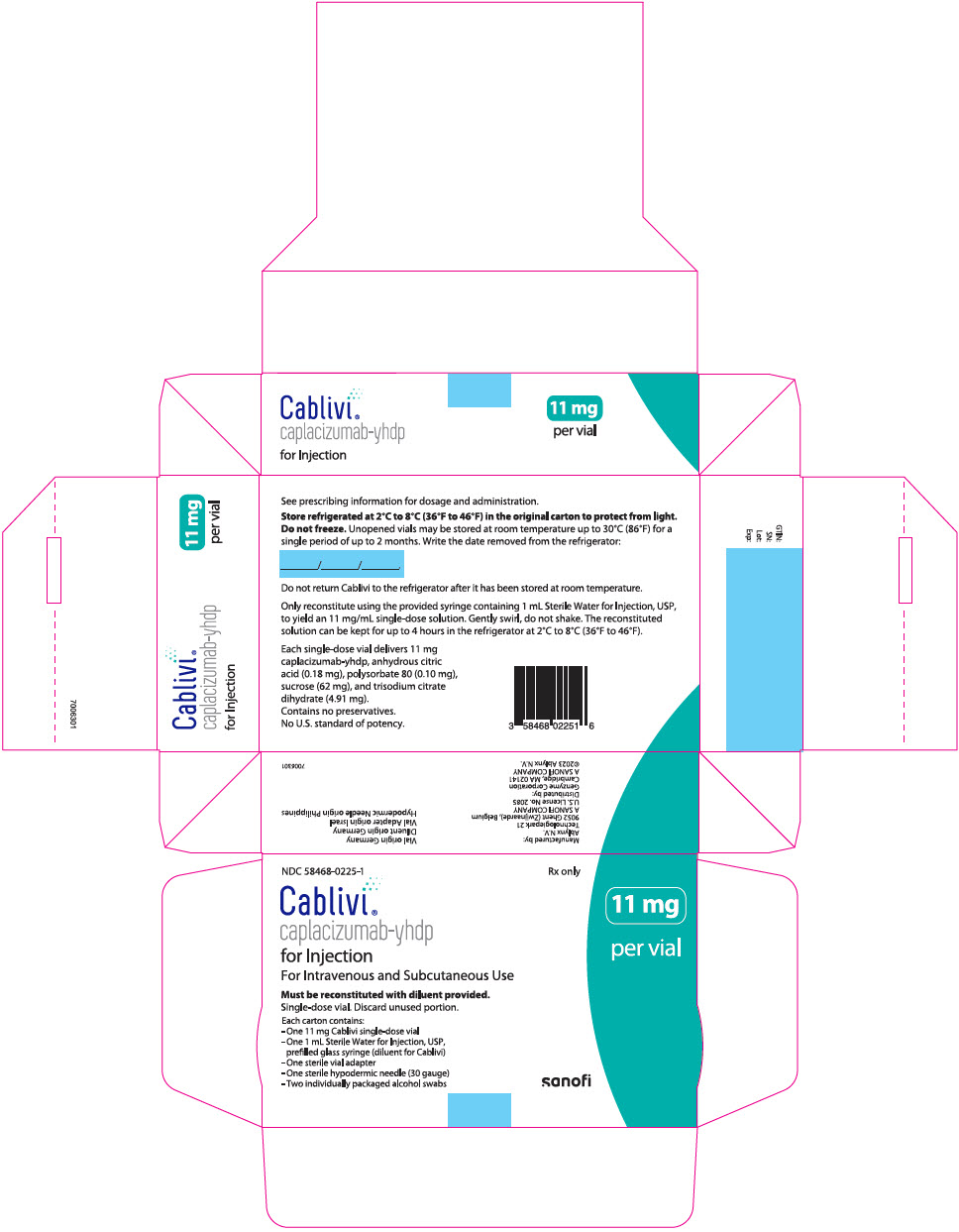
CABLIVI 11MG (11 MG) KIT 1X1 E
NDC No.58468-0225-01 58468-225-01 UPC/GTIN No..3-58468-22501-6 MPN 22501Only Lic.-Physician, Pharmacy, Dentist, Drug Mfg, Dist., Gov, Hospital, Lic.Lab, Naturalist, Naturopath, Np, Optometrist, Pharma

CABLIVI 11MG (11 MG) KIT 1X1 E
CABLIVI 11MG (11 MG) KIT 1X1 EA by SANOFI "Generic Name:
CAPLACIZUMAB-YHDP
Description:
CABLIVI 11MG (11 MG) KIT 1X1 EA
NDC:
58468-0225-01 58468-225-01 5846822501 58468022501
UPC:
358468-0

American Pharma Wholesale
Visit AmericanPharmaWholesale.com for over 100,000 items of Health & Beauty at Retail@Wholesale prices.

Buy More Save More!
Want to do Research on this Med or need a large quantity? Email Details with quantity required to:[email protected]

AmericanPharmaWholesale.com
Visit AmericanPharmaWholesale.com for over 100,000 items of Health & Beauty at Retail@Wholesale prices.

CABLIVI 11MG (11 MG) KIT 1X1 E
CABLIVI 11MG (11 MG) KIT 1X1 EA by SANOFI Generic Name: