
RAYALDEE- calcifediol capsule,
RAYALDEE- calcifediol capsule, extended release by Opko Pharma

AmericanPharmaWholesale.com
Visit AmericanPharmaWholesale.com for over 100,000 items of Health & Beauty at Retail@Wholesale prices.

NITROGLYCERIN SUBLINGUAL
Only Lic.-Physician,Pharmacy,Dentist,Drug Mfg,Dist.,Gov,Hospital,Lic.Lab,Naturalist,Naturopath,NP,Optometrist,Pharmacist,PA,Physical Therapist,Podiatrist,Research Co.,Uni.,VA,Vet & Wholesalers in scop

Buy More Save More!
Want to do Research on this Med or need a large quantity? Email Details with quantity required to:[email protected]

RAYALDEE- calcifediol capsule,
RAYALDEE- calcifediol capsule, extended release by Opko Pharma

AmericanPharmaWholesale.com
Visit AmericanPharmaWholesale.com for over 100,000 items of Health & Beauty at Retail@Wholesale prices.
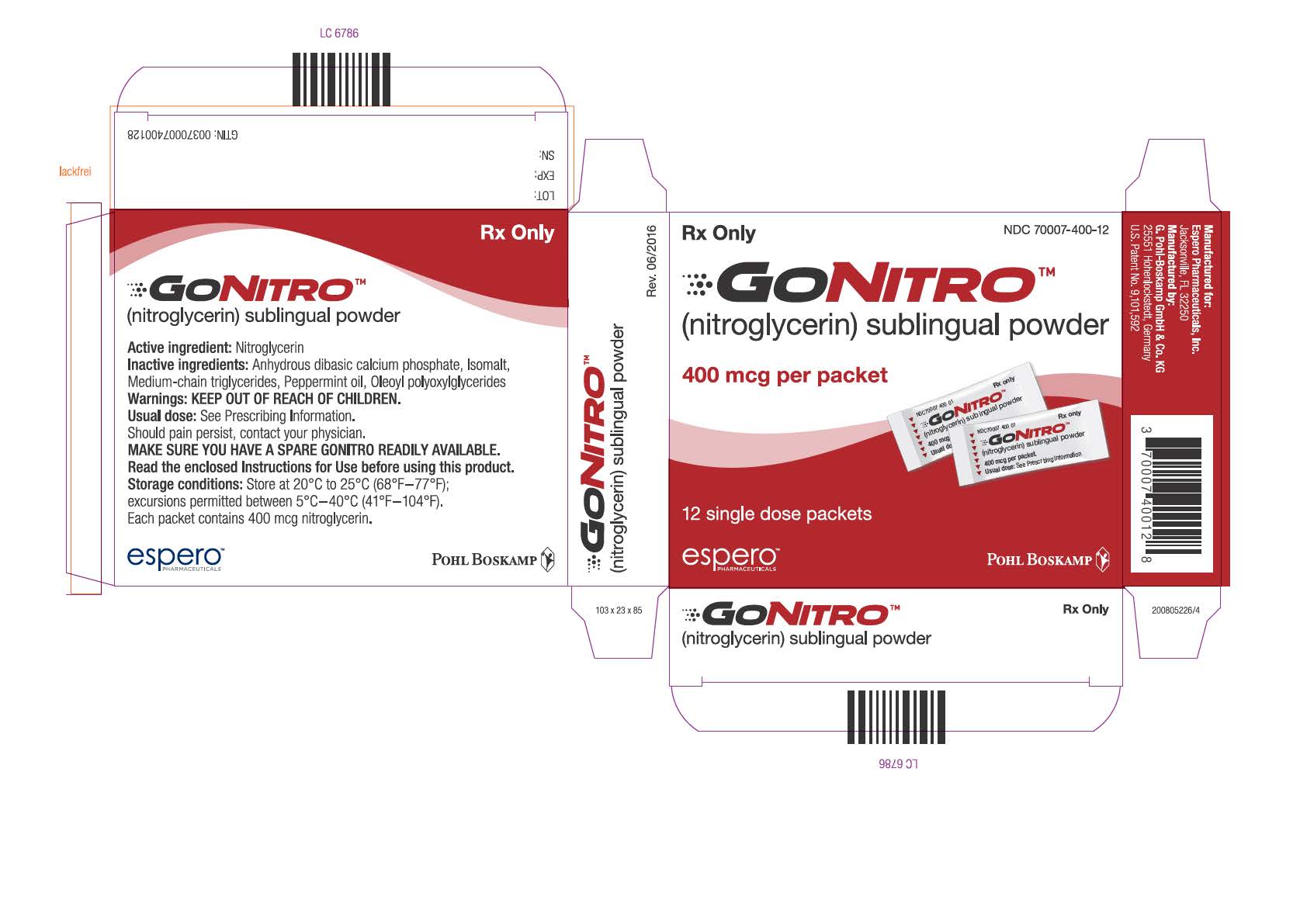
GONITRO nitroglycerin SUBLINGU
GONITRO nitroglycerin SUBLINGUAL 400MCG PWD 36 BY Allegis Pharma Item No.:RX598678 598678 NDC No.70007-0400-36 70007-400-36 7000740036 70007040036 UPC/GTIN No. 3-70007-40036-4 370007-400364 37000740

GONITRO nitroglycerin SUBLINGU
GONITRO nitroglycerin SUBLINGUAL 400MCG PWD 36 BY Allegis Pharma Item No.:RX598678 598678 NDC No.70007-0400-36 70007-400-36 7000740036 70007040036 UPC/GTIN No. 3-70007-40036-4 370007-400364 37000740